Nitric oxide may not be done yet. The colorless, odorless gas, inhaled through a mask or even from a small “flute,” is now being tested as an experimental treatment for COVID-19. It may also prove helpful in protecting healthcare workers on the front line of the pandemic from getting sick.
Medical researchers are testing whether inhaled nitric oxide could help treat patients with the coronavirus.
Researchers at Massachusetts General Hospital (MGH) are studying whether inhaling a gas called nitric oxide can help treat patients with COVID-19, or even prevent people from getting the disease.
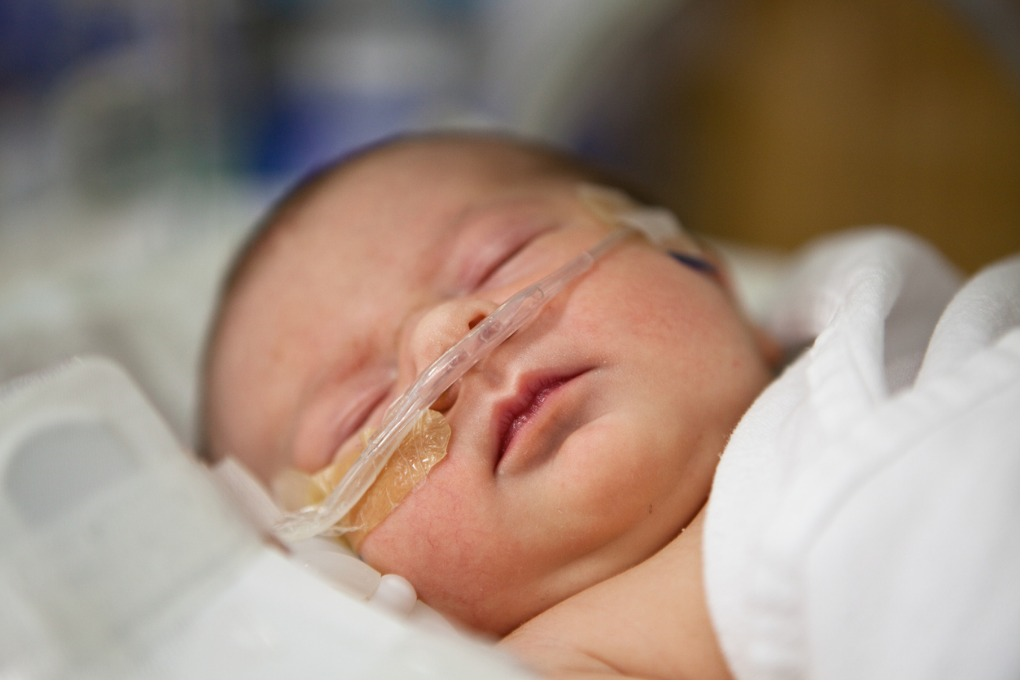
Nitric oxide has been considered a miracle drug for helping oxygen-starved newborns
A “remarkable” drug known to relax blood vessels could help coronavirus patients with severely damaged lungs and will be tested at Massachusetts General Hospital, making it the first center in the nation to rigorously test nitric oxide for novel coronavirus.
Critical care and emergency medicine faculty at the LSU Health Shreveport Department of Medicine join the Department of Anesthesia at Massachusetts General Hospital (MGH) and the Division of Cardiology in the Department of Medicine at University of Alabama-Birmingham (UAB) as being among the first centers in the United States to enroll patients in an international study testing using inhaled nitric oxide to improve outcomes for COVID-19 patients with severely damaged lungs; using gas to effectively “kill” coronavirus in the lungs and improve delivery of oxygen to injured tissues.
Doctors from around the world are seeing if the gas that gave us the ‘little blue pill’ will also help treat the novel coronavirus as it continues to spread.
Scientists in Massachusetts, Italy, and elsewhere are experimenting with a familiar medical treatment for the new problem of covid-19. They’ve begun clinical trials meant to find out whether inhaled nitric oxide can save people who are severely sick from the new coronavirus. This treatment might even be used preventatively to keep health care workers infection-free.
Never before, scientists say, have so many of the world’s researchers focused so urgently on a single topic. Nearly all other research has ground to a halt.
Arlington, MA – October 30, 2019 – Third Pole Therapeutics, a privately held company developing and delivering transformative life-saving cardio-pulmonary therapies, has named seasoned healthcare executive Bill Athenson as its new Chief Executive Officer.
Newest Patents Cover Nitric Oxide Generation and Wearable Delivery Systems
Arlington, MA – August 21, 2019 – Third Pole Therapeutics, a privately held company developing and delivering transformative life-saving cardio-pulmonary therapies, announced today that the U.S. Patent and Trademark Office has recently issued U.S. patents covering Third Pole’s innovative general and portable inhaled Nitric Oxide (iNO) delivery systems.
Treating pulmonary hypertension: New delivery system to change how babies receive nitric oxide therapy
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE
Each year, roughly 100,000 babies worldwide are diagnosed with a life-threatening condition called persistent pulmonary hypertension of the newborn (PPHN). In infants with PPHN, blood does not circulate properly through the lungs, and sufficient oxygen does not reach the heart, brain and other major organs. Yet, despite the proven effectiveness of inhaled nitric oxide (iNO) as a life-saving treatment for PPHN, access to it by patients has been severely limited—in part because of its expensive, unwieldy system of delivery…
Third Pole Therapeutics, a privately held company developing and delivering transformative cardio-pulmonary therapies, announced today that it has entered into a strategic collaboration to work with Actelion Pharmaceuticals Ltd, a Janssen pharmaceutical company of Johnson & Johnson…
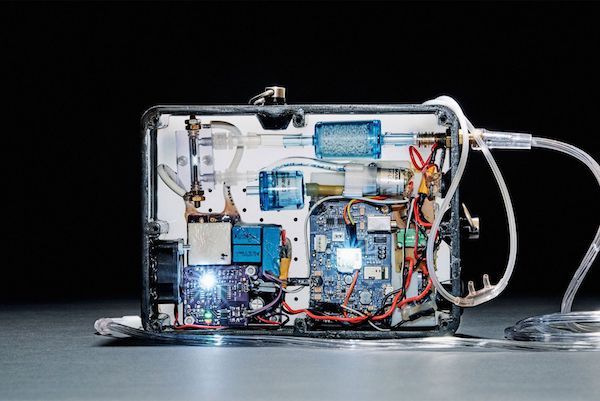
Third Pole Therapeutics said it has successfully completed an early feasibility study of its portable inhaled nitric oxide (iNO) delivery device, eNOfit, allowing the company to move to larger trials for pulmonary hypertension (PH) associated with interstitial lung disease.
WALTHAM, Mass., Feb. 27, 2024 (GLOBE NEWSWIRE) — Third Pole Therapeutics, a privately held company developing critical life-sustaining therapies for people living with cardiopulmonary and infectious diseases, announced today the successful completion of an early feasibility study (EFS) performed under an Investigational Device Exemption granted by the FDA. This study evaluated eNOfit™, a miniaturized, portable inhaled nitric oxide (iNO) generator …
– Data from Study Anticipated in Q1 2024 WALTHAM, Mass., December 11, 2023 (GLOBE NEWSWIRE) – Third Pole Therapeutics, Inc., Third Pole Therapeutics, a privately held company developing critical life-sustaining therapies for people living with cardiopulmonary and infectious diseases, today announced that the first patient has been dosed in an early feasibility study (EFS) performed under an Investigational Device Exemption granted …
Funding will support the final development phase prior to planned 2023 FDA submission of the company’s “breakthrough” designated eNOcare™ hospital device Waltham, MA –December 14, 2022 – Third Pole Therapeutics, a privately held company developing critical life-sustaining cardio-pulmonary therapies, announced today a $32M equity investment, from a large medical device innovator. Bill Athenson, CEO of Third Pole, stated, “We are …
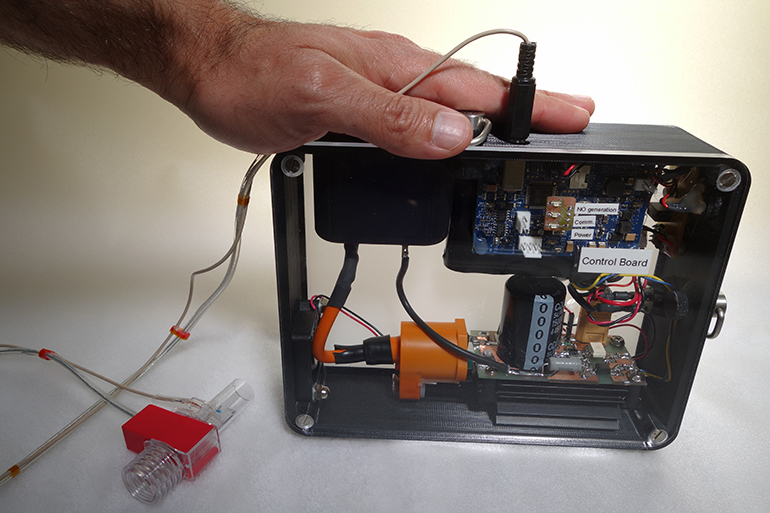
eNOfit™, Third Pole’s novel nitric oxide mobile-wearable device designed to provide “on-the-go” treatment for patients suffering from severe COPD and ILD, to enter clinical trials in the second half of this year Funding will support device optimization and clinical trials; Pivotal trial data expected in the second half of 2023 Waltham, MA – February X, 2022 – Third Pole Therapeutics, a privately …
WALTHAM, Mass., Jan. 6, 2022 /PRNewswire/ — Third Pole Therapeutics, a privately held company developing critical life-sustaining cardio-pulmonary therapies, announced today that it is presenting at the H.C. Wainwright BioConnect 2022 Conference and participating in BIO Partnering at JPM. All events are virtual and scheduled concurrently with the J.P. Morgan 40th Annual Healthcare Conference.
Bill Athenson, CEO of Third Pole Therapeutics, a privately held company developing critical life-sustaining cardio-pulmonary therapies, discusses eNOfit™ the company’s proprietary, tankless, on-demand, electrically generated inhaled Nitric Oxide (iNO) technology that is a safe, accurate and reliable lightweight, wearable device that makes iNO from electricity and the air we breathe, creating a make it and take therapy free from the hazards of compressed gas storage.
Last week the hospital marked a significant milestone – the 175th anniversary of the first public demonstration of ether in surgery, considered one of most game-changing events in medical history. In the spirit of that Oct. 16, 1846 landmark, the MGH, fittingly, shares the story of Courtney Joyce, who was the first patient at the hospital to benefit from inhaled …
Waltham, MA – April 15, 2021 – Third Pole Therapeutics, a privately held company developing critical life-sustaining cardio-pulmonary therapies, announced today that it has entered into a $15 million debt facility with Avenue Venture Opportunities Fund, L.P. (“Avenue Venture Fund”).
Waltham, MA – February 24, 2021 – Third Pole, Inc., a privately held company developing and delivering critical life-sustaining cardio-pulmonary therapies, announced today that it has added two seasoned pharmaceutical executives to its Board of Directors, William Heiden, former CEO and Director of AMAG Pharmaceuticals, and Werner Cautreels, Ph.D., former CEO of Selecta Biosciences.
The severity of COVID-19 respiratory failure in some patients has taken the medical community by surprise. And in response, that community has been trying just about anything that seems reasonable in an effort to improve outcomes — and in some cases as last-resort measures to save lives.
Waltham, MA – December 20, 2019 – Third Pole Therapeutics, a privately held company developing and delivering transformative life-saving cardio-pulmonary therapies, announced today its Chief Executive Officer Bill Athenson will present at Biotech Showcase 2020 to be held January 13-15, 2020 at the Hilton San Francisco Union Square.
Promising Prototypes to Watch in 2018
Engineering for Change
The technologies that improve lives in the world’s underserved communities are born as prototypes that evolve through testing and tinkering in workshops, laboratories and garages…
New device helps treat pulmonary hypertension
Digital Journal
A new device, the Third Pole is able to generate nitric oxide (NO) from the air that can help improve oxygenation and treat pulmonary hypertension a common problem among some newborns…
This Milk-Jug-Size Device Can Help Newborns Breathe
Bloomberg Businessweek
The Third Pole makes its own nitric oxide to treat pulmonary hypertension,
no tanks required….
Inhaled nitric oxide (iNO) relaxes blood vessels in the lungs and is an important and life-saving treatment for pulmonary hypertension. Current iNO delivery solutions are estimated to cost $2,800 per day and rely on compressed gas delivery which limits accessibility and applicability of this technology worldwide…
Third Pole’s Lighter Nitric Oxide System Wins Johnson & Johnson Innovation Award
Pulmonary Hypertension News
Third Pole has won a Johnson & Johnson Innovation award for a lighter device that provides an unlimited supply of nitric oxide (NO) to patients with pulmonary hypertension (PH) and other respiratory conditions…
Nitric Oxide
Volume 60, 2016, Pages 16-23
ISSN 1089-8603
Authors:
Binglan Yu, Aron H. Blaesi, Noel Casey, Grigory Raykhtsaum, Luca Zazzeron, Rosemary Jones, Alexander Morrese, Danil Dobrynin, Rajeev Malhotra, Donald B. Bloch, Lee E. Goldstein, Warren M. Zapol
Science Translational Medicine
Vol. 7, Issue 294, pp. 294ra107
Authors:
Binglan Yu, Stefan Muenster, Aron H. Blaesi, Donald B. Bloch,
and Warren M. Zapol
Investigator Profile: Warren Zapol, MD and Nitric Oxide
Partners Innovation
In the 25 years since discovering a life-saving therapy consisting of breathing low levels of the gas nitric oxide—which is still the only selective inhaled pulmonary vasodilator—Massachusetts General Hospital (MGH) anesthesiologist Warren M. Zapol, MD, and his MGH team has given clinicians a treatment to simply save the lives of vast numbers of hypoxic newborns (“blue babies”)and prevent or reverse pulmonary hypertension in hundreds of thousands of adults during surgery…